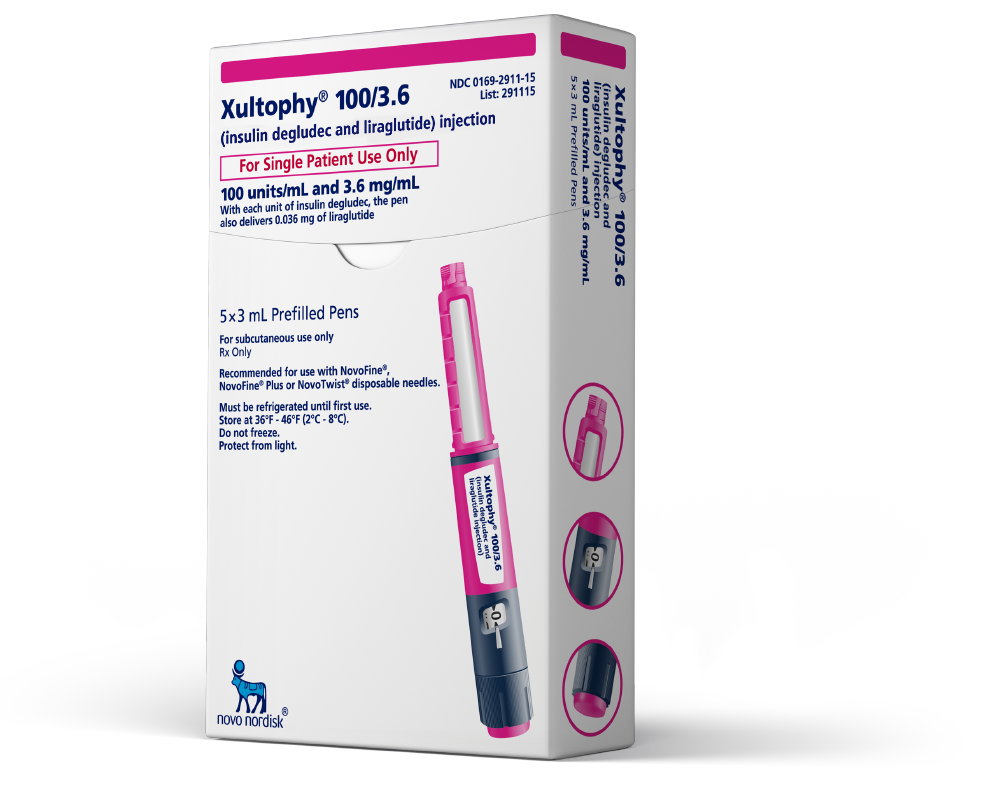
Novo Nordisk Receives FDA Approval for Xultophy® 100/3.6 (insulin degludec and liraglutide injection)

How to Prescribe Xultophy® | Xultophy® 100/3.6 (insulin degludec and liraglutide) injection 100 u/mL and 3.6 mg/mL
Xultophy® 100/3.6 Savings, Savings Card & More | Xultophy® 100/3.6 (insulin degludec and liraglutide) injection 100 units/mL and 3.6 mg/mL

Novo Nordisk issues voluntary nationwide recall of Levemir®, Tresiba®, Fiasp®, Novolog® and Xultophy® product samples due to improper storage temperature conditions

Xultophy® 100/3.6 HCP Website | Xultophy® 100/3.6 (insulin degludec and liraglutide) injection 100 u/mL and 3.6 mg/mL

These highlights do not include all the information needed to use XULTOPHY 100/3.6 safely and effectively. See full prescribing information for XULTOPHY 100/3.6. XULTOPHY® 100/3.6 (insulin degludec and liraglutide) injection, for subcutaneous